(Sponsored post)
Protection against microbiological contamination
We can define a cosmetic product as a prepared mixture based on various natural or synthetic chemical substances intended for hygiene or care of external parts of the human body. That’s before modifying their structure or functions, to protect or decorate them or create well-being. Ingredients incorporated as raw materials into the formulations of these products are also considered cosmetic products.
When we acquire, handle, and use cosmetic and personal care products, above all, we tend to value how they feel. Therefore, the physical and emotional sensations of finished products are considered by manufacturers just as much as defining their formulation, presentation, and ingredient selection. With that said, cosmetic preservatives play a dual role in this context: both in technical protection and marketing.
Safe preservation of cosmetics
Due to the nature of their composition and the conditions in which they are used, many cosmetic products are vulnerable to the intrusion of microorganisms and may even break down when contamination develops. The current systems for preservation of cosmetic formulations are designed by simultaneously combining several factors: the formula ingredients, manufacturing process, packaging and handling, final presentation of the product, and the way it will be used.
Preservatives for cosmetics must be able to inhibit the unwanted microbial growth that can occur inside the container before the preferred use date, while it remains closed, and once it’s been opened. They must also avoid the development of contamination through the entire life cycle of the cosmetic. Furthermore, like all cosmetic ingredients, preservatives must be safe when they come into contact with the skin.
For the preservation system to achieve its protective function, the antimicrobial ingredient selection process must be based on previous analyses evaluating the risk of contamination of each product. In addition to the use of preservatives in cosmetics, it is also vital to apply primary microbiological control concepts, such as implementing an effective hygiene plan within the production plant.
The Chemipol microbiology team determines the level of contamination risk by using a matrix to evaluate the microbiological risk for all the parameters that most favour the growth of microorganisms. These values are:
- The free water content in the formula, as that is where the microorganisms thrive.
- The content of appropriate nutrients in the form of organic matter and microelements.
- The temperature, pH, and oxygen content parameters, as these three factors favour the development of colonies of microorganisms.
Based on the risk indicated by the valuation matrix, we can apply a specific preservation strategy for each product. This can be achieved by selecting preservatives for cosmetics themselves formulated with optimal combinations of different substances that have inhibitory action of the contamination, as well as ingredients with antimicrobial activity and efficacy enhancers. The aim of this process is to transform the cosmetic formulation into a product that is not easily contaminated and that, therefore, does not decompose due to the effect of microorganisms.
The composition of these new preservation systems is balanced in such a way that it represents a barrier to the development of microorganisms in the cosmetic product formulation itself. It will also reduce the accumulated toxicity of the finished cosmetic product by reducing the use of traditional preservatives.
Antimicrobial barrier
Certain physicochemical properties of cosmetic formulations, the use of raw materials with bacteriostatic and fungistatic effects as secondary characteristic, and correct handling and production methodology can configure sufficient ‘self-protective’ conditions for the preservation of cosmetics. This means that the use of traditional biocides (listed as preservatives by the regulations) becomes unnecessary.
The six steps to building an effective antimicrobial barrier
The first step is to keep the pH as low as possible to limit the possible variety of viable microorganisms. Each species survives in a specific pH range, therefore conditions outside the thresholds of what the strain can tolerate will mean that these microorganisms will be unable to function, making them unviable. To reduce the pH, acids that are not listed as preservatives for cosmetics (citric acid, lactic acid, and sodium citrate, among others) can be incorporated into the formula.
The second step is to reduce the available water (Aw). This parameter should not be understood as the total water content but rather, as the formula water not used in the solubilisation of another ingredient and as the relationship between the vapor pressure of a substance or formulation and pure water at the same temperature. As a reference, pure water has an Aw value of 1. This parameter limits the growth of microorganisms since fungi and yeasts can develop at Aw values around 0.6-0.8, Gram negative bacteria require Aw levels in excess of 0.9, and Gram-positive bacteria require an Aw content above of 0.8. The use of glycerine and its derivatives, or glycols, polyalcohols, and solubilising agents such as polysorbate (Tween 20) can lower the Aw, thus helping to control the development of Gram-negative bacteria which are extremely sensitive to this value.
The third step is to reduce the concentration of metal ions and cations in the medium because these can contribute to forming the cell walls of some microorganisms; in some cases, they can even inhibit the action of certain cosmetic preservatives such as organic acids. To eliminate metal ions and cations that might be present in microorganism cell walls, chelating ingredients such as EDTA, NTA, or glutamic derivatives, among others, can be used in the formulation of the cosmetic product.
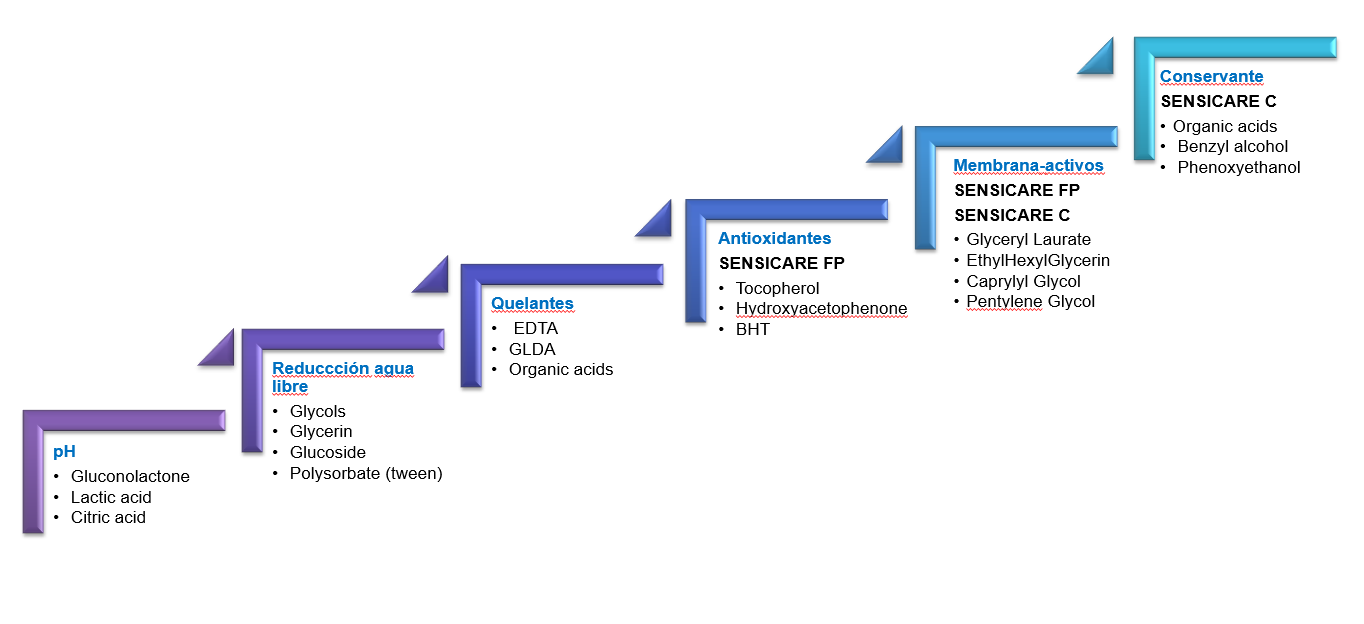
The fourth step is the reduction of free oxygen present in the formulation. The presence of oxygen is essential for the growth of any aerobic microbe. Thus, the use of antioxidants such as free oxygen scavengers and stabilisers will make it difficult for microorganisms to obtain this molecule. The antimicrobials for cosmetics contained in the SENSICARE® FP range incorporate antioxidants such as hydroxyacetophenone, while those in the SENSICARE® NAT range contain vitamin C with natural extracts that enhance this barrier effect.
The fifth step is the use of antimicrobial efficacy enhancers or ‘boosters’ that increase interactions with microorganism cell membranes. These ingredients are raw materials not classified as preservatives for cosmetics but, due to their lipophilic/hydrophilic balance, they can absorb the lipids that form microbial cell walls. This disrupts the proper functioning of the membrane by creating gaps in it, thereby allowing the preservative ingredients to penetrate them. In addition, the permeability of the part of the membrane not directly affected by these openings may also increase. The efficacy-enhancing or ‘boosting’ ingredients most used in the SENSICARE® range are based on polyalcohols and glycerine derivatives.
The sixth step is the use of antimicrobial agents or preservatives for cosmetics. The use of preservatives listed in cosmetic regulations can be avoided altogether by applying the previous steps and by incorporating antimicrobial substances and efficacy enhancers. Examples of these are the products used in the SENSICARE® FP and SENSICARE® NAT ranges, which are formulated entirely with ingredients not listed as preservatives.
However, in cases where the microbiological risk assessment matrix indicates an elevated risk of contamination, it may not be possible to preserve the cosmetic product through the antimicrobial barrier alone. In these cases, the product should be protected with some of the cosmetic preservatives based on traditional ingredients which already incorporate efficacy enhancers. Examples include SENSICARE® C 1000 and SENSICARE® C 3300, which use phenoxyethanol or benzyl alcohol with boosters, or mixtures of organic acids and their salts with boosters such as SENSICARE® C 2050, SENSICARE® C 2060, and SENSICARE® C 2060.
Improvement of the toxicological profile and different marketing options
In addition to their main protective function against microbial growth, the new preservatives and antimicrobial additives for cosmetics also make it possible to satisfy all the marketing criteria that are considered when positioning cosmetic and personal care products. Using preservatives for traditional cosmetics makes it possible to protect cosmetic formulations that have a considerable risk of microbiological contamination or whose positioning and marketing channels require low sales prices.
Antimicrobial additives that create an inhibitory barrier effect against microbiological development have different mechanisms of action on microorganisms. This contributes to a broad-spectrum protection and helps to improve cosmetic formulations in two ways. On the one hand, they do not increase the sensitising capacity or toxicity of the cosmetic formulation, while on the other, they allow companies to market preservative-free products for cosmetics because the ingredients used are not classified as such.
Conclusions
New preservation technologies protect cosmetic products against microbiological contamination throughout their useful lifespans by building an antimicrobial barrier. They do this through a combination of cosmetic preservatives, antimicrobial additives, and efficacy enhancing ingredients known as boosters that together, inhibit the growth of microorganisms. The incorporation of these ingredients does not increase the sensitising capacity or aggravate the toxicological classification of the formulation, which in turn allows a variety of marketing strategies to be applied.

by Miquel Ramírez, head of the cosmetics business unit, and Eduard Broto, sales manager.

