One of the dilemmas of any formulator, chemist or brand owner is to ensure they choose the right ingredients in compliance with the regulatory criteria for the relevant market in the foreseeable future to avoid reformulating costs and supply chain nightmares.
On the other hand, the regulatory landscape is ever changing due to new knowledge in the field of toxicology and developing new tests’ methods to evaluate ingredients’ safety.
In this article, we will discuss the substances that are being restricted in the EU zone (27 countries) while being placed on the market in 2022.
Based on the EU regulatory format for updating ingredients’ safety, any new information will be submitted to the Scientific Committee on Consumer Safety (SCCS). This committee consists of several experienced experts in the field of human safety and toxicology and provides opinions on consumers’ safety based on the latest information. The committee usually provides its reports in response to a specific request.
The EU Commission, in 2020, committed to creating a priority list of potential endocrine disruptors that weren’t covered yet by the regulation bans under substances classified as carcinogenic, mutagenic, or toxic for reproduction (CMR). After input from EU countries, the industry, consumer organisations and the SCCS, the Commission consolidated a list of 28 substances. Many of substances in this list were reviewed by the SCCS based on the latest available data. Hence, we are expected to have several new restrictions in 2022.
Dihydroxyacetone (DHA): One of the hot topics in the regulatory field in 2022 is the restriction in concentration of this ingredient which affects self-tanning products. Dihydroxyacetone is the aliphatic ketone, known in the industry as DHA. It is a common ingredient that may be found in many tanning and hair dye products.
This ingredient is not known to be a skin sensitiser or irritant to eyes and has a low acute toxicity profile. In 2008, The Scientific Committee on Consumer Safety (SCCS) concluded that, based on available data, the use of DHA as self-tanning ingredients can be considered as safe by 10% and safe up to 14% in the spray cabin.
However, considering the new data submitted to the commissioner in 2019, the SCCS has reviewed its previous opinion and came to the conclusion that the use of DHA as hair colourant in non-oxidative hair products (leave on) is safe to maximum concentration on 6.25% together with self-tanning lotion and face cream to a maximum of 10% in finished products.
This opinion turned into regulation from 26.02.2022 and there should be NO product available on the market from 22nd May 2022 that contains DHA concentration above the set criteria. This regulation is only applicable in the EU and Northern Ireland and is not yet confirmed in the UK.
Methyl salicylate: It is the ester of methyl alcohol and salicylic acid and it is mainly used in cosmetic products as denaturant, flavouring, oral care, perfuming and soothing. Based on new evaluation and recent studies, the SCCS (SCCS 1633/21) has considered this ingredient as a skin sensitiser.
Since metabolic products of Methyl salicylate in the body are a salicylic acid and this ingredient is considered as a CMR 2 (reprotoxic); hence the accumulative exposure to cosmetic products containing various salicylates may impact the final safe level for consumers.
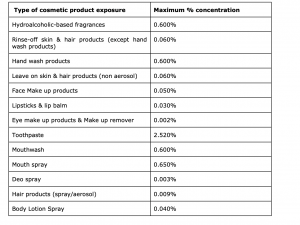
Based on this opinion, there will be a new restriction for this ingredient in various products placed on the market from Dec 2022. The below table will explain the new restriction based on each cosmetic category:
Benzophenone-3 and Octocrylene: UV filters used in cosmetic products follow the restriction set in annexe VI. If an ingredient is not listed in annex VI, it cannot be used in a formulation as a UV filter. From the annex VI, these two ingredients were listed in the EU commission priority list A for immediate review in 2021.
Octocrylene: It is the substituted acrylate listed under Annex VI (10), a light stabiliser and UV filter. Based on the recent SCCS review (SCCS 1627/21), the Committee did not consider the current evidence for octocrylene endocrine effect sufficient. The reported contact sensitiser for this ingredient was evaluated as negligible due to its widespread use and considered it as negligible.
Based on cumulative exposure, the SCCS restricted the use of this ingredient in propellant spray to 9% (from previous 10%) when used together with face cream, lip cream and hand cream containing 10% Octocrylene.
Benzophenone-3 (BP-3): This ingredient is a benzophenone derivative listed under UV filter (Annex VI/4) and as a light stabiliser function (not in annex VI). The SCCS did not consider the presented evidence as sufficient to list this substance under CMR category. However, the committee warrants further investigation. Previously this ingredient was allowed to be used by 6% (2017/238) and not more than 0,5 % to protect product formulation. Based on the new SCCS opinion (1625/20), the new restrictions are:
-Face products, hand products, and lip products, excluding propellant and pump spray products (6%: unchanged)
-Body products, including propellant and pump spray products (limited to 2.2%)
The limitation for its concentration for any other function remains at 0.5% and the warning statement should be on the label (no change on this statement) as: Contains Benzophenone-3
The restriction for both BP-3 and Octocrylene are expected to take place from third quarter in 2022.
Methyl-N-methylanthranilate (M-N-MA): It is a fragrance ingredient used in various cosmetics, including fine fragrances, shampoos, soaps, and other toiletries. Previously it was considered as safe at concentration up to 0.2% in rinse-off and 0.1% at leave-on products.
The safety concern for this ingredient was raised when being used on areas exposed to light. M-N-MA has phototoxic properties but based on the provided data using this substance with concentrations from 0.1% to 0.5% with a UV dose that realistically represents skin exposure during outdoor activities (excluding sunbathing) should not result into any phototoxic reactions.
However, the SCCS (SCCS/1616/20) did not consider this ingredient as safe when being used in sunscreen products and products marketed for exposure to natural/artificial UV light, due to possibilities of prolonged exposure. This restriction will come into place in the third quarter in 2022.
In the next part of this article, we will discuss the substances that are to be banned in 2022.
Personal Care Regulatory Ltd make sure to track ingredients restriction and update their clients’ safety assessment reports on time and regularly.
Get in touch with them for further discussion at: info@personalcareregulatory.com
Enjoyed this article? Get more by subscribing to our newsletter!
Feeling inspired to see ingredients and trends in action?
Then why not visit one of the in-cosmetics events around the world?

