In the recent non-special use cosmetics (Non-SUC) filing process in China, the review of trademarks in foreign languages has become stricter. We collected the most common review issues on trademarks and combined with relevant laws and regulations to summarize the Chinese labelling requirements of foreign trademarks.
Non-SUC Filing Review Opinions on Trademarks
1) Beijing MPA

Review Opinions
According to the latest instructions of the NMPA, whether it is the English trademark in the product name or on the label, the following Chinese interpretation must be labelled:
- **是注册商标,其含义是** (** is a registered trademark, and its meaning is **)
- **仅为注册商标,无具体含义 (** is only a registered trademark and has no specific meaning.)
Only marking ® on the trademark or only submitting the trademark registration certificate will fail the filing review. All the English on the label must be explained in Chinese.
Failure Reasons
English trademarks without Chinese interpretation.
2) Shanghai MPA
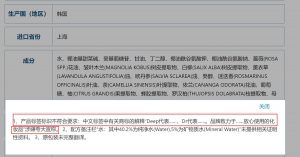
Review Opinions
The interpretation of the trademark in the Chinese label contains exaggerated claims: “Dr. Deep” is the product brand name and a registered trademark. “Deep” represents that the product quality is worthy of deep trust. “Dr” represents the product is so professional that can be a doctor of family skincare. The brand is committed to selecting high-quality ingredients and developing safe and reliable cosmetics.
Failure Reasons
Trademark interpretation contains non-compliant claims.
3) Guangdong MPA
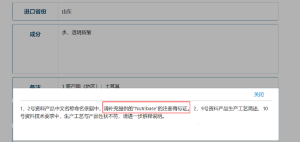
Review Opinions
Please provide the trademark registration certificate of “Nutribase” in the product name.
Failure Reasons
Without a valid trademark registration certificate.
Regulations on Trademark Labeling
CSAR Subsidiary Regulation: Administrative Measures on Cosmetics Labeling (Draft for Comments)
Article 7 (Requirements for Label Text) Standard Chinese characters shall be used in cosmetic labels. If other languages or symbols are used, standard Chinese characters shall be used on the product sales packaging to provide corresponding interpretations, except for websites, name and address of overseas enterprises, and conventional professional terms that must use other languages.
Article 8 (Requirements for Trade Name, Generic Name and Attribute Name) The name of a cosmetic product generally consists of three parts: a trade name, a generic name, and an attribute name, all of which shall meet the following requirements:
…
(II) The medical effect or the efficacy that the product does not have shall not be claimed by a trade name. Where the name of the ingredient or a term implying that it contains a certain ingredient is used as the trade name, and the ingredient appears in the product formula, the purpose of use shall be explained in the visual panel of the sales packaging; and the ingredient does not appear in the product formula, it shall be clearly labelled on the visible panel of the sales packaging that the product does not contain such ingredient, and the ingredient name is only used as a trade name.
Article 9 (Requirements for Labeling of Product Name)
Chinese names of cosmetics must not be named using letters, Chinese Pinyin, figures, symbols, etc., except for registered trademarks, sunscreen index, color numbers, serial numbers, or other letters or symbols that must be used. Where the letters, Chinese Pinyin, figures, symbols, etc. are used in the registered trademark in the Chinese name of a product, the meaning thereof shall be explained in the product label.
Labelling Requirements of Foreign Trademarks
Based on the above review opinions and regulations, trademarks in foreign languages should meet the following requirements.
1) Foreign trademarks must provide a valid trademark registration certificate during cosmetics filing or registration. Only marking ® on the trademark or only submitting the trademark registration certificate will fail the filing review. The following Chinese interpretation must be labelled on the packaging:
- For registered trademarks with specific meanings, shall label “**是注册商标,其含义是**” (** is a registered trademark, and its meaning is **).
Examples:

LYMPHODIA is a registered trademark. “LYM” is the abbreviation of “Love You More”, which means to love you more. “PHO” means bright, and “DIA” is the abbreviation of the diamond. The overall meaning of the trademark is: based on the concept of love, our brand cares for the skin and brings a diamond-like lustre for the skin.
- For registered trademarks without specific meanings, shall label “**仅为注册商标,无具体含义” (** is only a registered trademark and has no specific meaning).
Examples:

P. Jentschura is the name of the brand founder, Peter Jentschura. P is an abbreviation of Peter, with no specific meaning.
1) Trademark interpretation must not contain non-compliant claims;
2) Pay attention to the new requirements in the draft labelling regulation. Where the ingredient name or a term implying an ingredient is used as the trade name:
- For products containing that ingredient, the purpose of use should be labelled on the packaging;
- For products without that ingredient, it shall be labelled on the packaging that the product does not contain such ingredient, and the ingredient name is only used as a trade name.
The new cosmetic overarching regulation CSAR will be implemented on Jan. 1, 2021, bringing more stringent requirements for cosmetics market entry and heavier penalties for non-compliance. We recommend you stay in touch with any CSAR updates, which you can find out more about here.
 ChemLinked is a leading provider of Asia-Pacific regulatory information and market intelligence across Cosmetic, Chemical, Food and Agrochemical Industries. ChemLinked boasts a multidisciplinary team of scientists, compliance specialists and language experts backed by the vastly experienced technical teams at REACH24H, to ensure over 44,000 registered members to acquire authoritative information and dependable consultancy services. ChemLinked aims to remove any regulatory barriers that prevent you from exporting your products into China and other countries in the Asia-Pacific region
ChemLinked is a leading provider of Asia-Pacific regulatory information and market intelligence across Cosmetic, Chemical, Food and Agrochemical Industries. ChemLinked boasts a multidisciplinary team of scientists, compliance specialists and language experts backed by the vastly experienced technical teams at REACH24H, to ensure over 44,000 registered members to acquire authoritative information and dependable consultancy services. ChemLinked aims to remove any regulatory barriers that prevent you from exporting your products into China and other countries in the Asia-Pacific region
- LinkedIn: https://www.linkedin.com/company/chemlinked/
- Facebook: https://www.facebook.com/chemlinked/
- Twitter: https://twitter.com/ChemLinked

