Lyotropic liquid crystals can form when compounds containing lipophilic (fat-loving) and hydrophilic (water-loving) structures known as amphiphiles dissolve into a solution. They are often present in water and oil interfaces and help lower the interface surface tension. Lyotropic liquid crystals thereby contribute to the formation of emulsions, which are the essence of most cosmetic formulations.
The micro-phase segregation of two incompatible parts in the same molecule results in different types of solvent-induced, extended anisotropic arrangements; the characteristics of these arrangements depend on the volume balances between the hydrophilic part and the lipophilic part. These anisotropic arrangements generate a long-range phase order, with the solvent molecules filling the space around the compounds to provide fluidity to the system.
Lyotropic liquid crystals have a unique ability to induce a variety of different phases at different concentrations. As the concentration of amphiphilic molecules increases, several different types of lyotropic liquid crystal structures occur in solution. Each of these different liquid crystal structures has a different extent of molecular ordering within the solvent matrix, from spherical micelles to larger cylinders, aligned cylinders, and bilayer and multiwall aggregates.
In most lyotropic liquid crystal systems, aggregation occurs only when the concentration of the amphiphile exceeds a critical concentration, variably referred to as the critical micelle concentration (CMC) or the critical aggregation concentration (CAC).
At very low amphiphile concentrations, the molecules are dispersed randomly and without any ordering. At slightly higher concentrations just above the CMC, self-assembled amphiphile aggregates exist as independent entities in equilibrium with monomeric amphiphiles in solution, but they exhibit no long-range orientational or positional order; the phases are isotropic and not liquid crystalline. These dispersions are referred to as micellar solutions, denoted by the symbol L1. The spherical aggregates within are known as micelles.
At higher concentrations, the assemblies become ordered. And when the concentration of amphiphile in water is increased beyond the point where the micellar aggregates are forced to be arranged regularly in space, lyotropic liquid crystalline phases form. The concentrations at which this formation occurs depends heavily on the chemical compositions of the amphiphiles, but it normally ranges from 5-30% by weight.
Liquid crystal structures are known to impact the stability and skin feel of cosmetic formulations. Therefore, they are fundamental to the production of physically stable emulsions that display desirable rheological profiles for pleasant skin application. We will detail the relationship between liquid crystal structures, emulsion stability, and skin feel in our future contributions; in this article, we will simply explain in principle how best to maximize the formation of liquid crystals when formulating an emulsion cosmetic product.
A typical phase diagram for an amphiphile emulsifier (as a function of concentration in water and temperature) is shown in Figure 1. It is known that the lamellar structure, denoted by the symbol Lα or Lβ, is particularly beneficial to the stability and skin feel of the resulting emulsion (Figure 2). However, with most emulsifier molecules, the lamellar liquid crystal structure only forms at relatively high concentrations (> 5%) in water, as illustrated in Figure 1. Such high levels of emulsifier molecules severely limit the formulation flexibility of the overall product; this often causes cosmetic products to have poor performance or undesirable skin feel, not to mention the exorbitant cost usually associated with adding high amounts of emulsifiers.
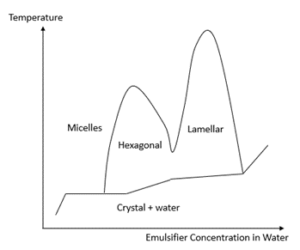
Figure 1 – A simplified phase diagram of an emulsifier molecule.
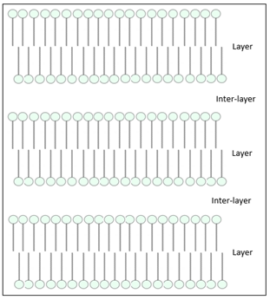
Figure 2. Illustration of an emulsifier lamellar liquid crystal structure.
To overcome this challenge, a stepwise formulation make-up process can be used. First, a stable intermediate is made with a concentration of the emulsifier molecule that is high enough to ensure the production of large quantities of lamellar liquid crystals. Then, appropriate amounts of oil and water are added to the intermediate to reach the target emulsion composition. This strategy is illustrated using a ternary isothermal phase diagram in Figure 3.
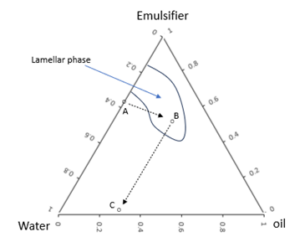
Figure 3. Ternary isothermal phase diagram that illustrates the stepwise formulation strategy.
In Figure 3, to reach the target composition (C) with eventual emulsifier loading of < 5%, we start with an emulsifier water solution with > 60% emulsifier molecule (A). Oil is then added to this mixture to reach the intermediate (B). It is critical that the intermediate is within the lamellar phase so that the maximum amount of lamellar liquid crystals is formed. Finally, appropriate amounts of water and oil are added to the intermediate to reach the target composition (C).
To make this strategy work, the emulsifier molecules need to have the correct structure to form strong lamellar liquid crystals. Furthermore, the oil chosen should have a structure that does not negatively impact the lamellar liquid crystal arrangement. Lastly, the addition of water should ideally just increase the inter-layer space in the overall lamellar arrangement. The chemistry needed to satisfy all these requirements will be the topic of our future articles.
Enjoyed this article? Get more by subscribing to our newsletter!
Feeling inspired to see ingredients and trends in action?
Then why not visit one of the in-cosmetics events around the world?

