Molecular mesophase behavior originates from two basic concepts. The first concept is the “closest packing” of simple-shaped mesogenic compounds. In these shape-driven mesophases, rod-like molecules are packed in an ordered liquid crystal phase. This ordered liquid crystal phase is energetically more stable than the randomly-packed isotropic phase. Most of the classical liquid crystals, notably the compounds used for traditional display devices, belong to this class: the monophilic liquid crystal.
The second concept is the “microphase separation” of two incompatible parts within one molecule. The most common incompatible characters are hydrophilicity and lipophilicity coexisting in one molecule, which is thereby amphiphilic. Amphiphilic molecules can form amphiphilic liquid crystals.
For example, glycolipids consisting of two incompatible parts—a polar sugar residue which is hydrophilic and a nonpolar paraffin chain which is lipophilic—display double melting behavior, which is characteristic of liquid crystal formation. The two incompatible parts of this molecule are connected covalently and cannot separate macroscopically. Instead, the molecules are forced to microscopically separate into a sequence of hydrophilic and lipophilic layers. These layers have the same geometry as the smectic A phase of the monophilic liquid crystals; as such, their physical properties are also the same. While monophilic liquid crystals and amphiphilic liquid crystals share many properties associated with their liquid crystallinity (e.g. textures of the smectic A phases, X-ray patterns in the mesophases, etc.), the chemical requirements for the formation of these two types of liquid crystals are completely different.
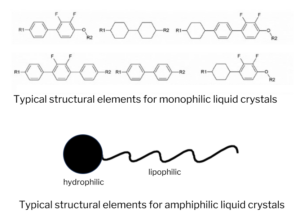
Many amphiphilic molecules can form mesophases both in melt (thermotropic liquid crystals) and in solution (lyotropic liquid crystals). Some researchers use the term amphotropic liquid crystals to express the ability of a compound to form both thermotropic and lyotropic mesophases.
Amphiphilic molecules with mesophases exhibit many useful properties for technological applications. Common applications include coating medicines to control their delivery, stabilizing hydrocarbon foam, and serving as primary solvents for topical medication.
Thermotropic properties of amphiphilic molecules are determined by several factors, though the amphiphilic character is the most important one. Some of these factors are discussed below:
- A mesogen must consist of at least two different molecular parts which do not like to mix with each other; these parts can be hydrophilic and lipophilic, hydrophilic and fluorinated, siloxane and hydrophilic, etc. The contrast in attraction forces between these two molecular parts is one of the main driving forces for the formation of these mesophases.
- The sizes of the molecular parts are If we only consider the difference in hydrophilicity, methanol (CH3OH) looks like an amphiphilic molecule. However, the polar and nonpolar moieties in methanol are too small. As such, the ratio between volume and surface of the microphases is too small to display any amphiphilicity.
- The balance between hydrophilicity and hydrophobicity in the molecular structure should be appropriately adjusted. There is an optimum lipid chain number for a given polar head group. For example, polar monosaccharides with 4 OH groups attain their highest clearing temperatures when attached to 16-carbon alkyl lipophilic chains.
- The relative positions of the two different parts within the molecule is important. Molecular fluidity such as that in the liquid phase (e.g. translations, rotations) should be possible in a mesophase, yet the two different molecular parts should be clearly separated. This is possible with rod-like or rope-like molecules, which form lamellar arrangements. It is also possible with wedge-shaped molecules, which form columnar phases. Many other possible arrangements of the two different parts may cause amphiphilicity but will not yield the formation of liquid crystals.
- There should be flexible parts of the molecule. For example, a mesogenic compound with only phenyl rings and a sugar moiety is hindered in the formation of thermotropic mesophases.
- We now know that the exact stereochemistry of the molecule influences its thermotropic properties; however, not all of the details have been thoroughly researched yet.
Lyotropic liquid crystal behavior is similar to that of thermotropic liquid crystals. However, its liquid crystal phase changes continuously with changes in the amount of solvent as well as temperature; therefore, a lyotropic system has one more variational vector than a thermotropic system. In addition, lyotropic liquid crystal behavior can be influenced by the types of solvents and their concentrations.
With lyotropic liquid crystals, the textures seen under a microscope with crossed polarized light are normally more complicated. The cubic mesophases tend to only display a “black” texture. As such, while microscopy is suitable for observing the thermotropic mesophases, X-ray analysis is commonly used to study lyotropic properties, especially the cubic phases.
The relationship between molecular shapes and their expected mesophase behavior is illustrated below, though, as previously discussed, the actual phase of a liquid crystal is further determined by its temperature and solvent environment.
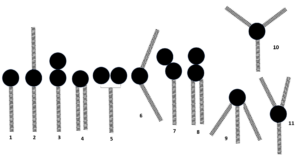
Elongated amphiphiles with structures like 1, 2, and 3 tend to form a lamellar phase. Forked (4) or pie shaped (5) mesogens will most likely form hexagonal columnar phases. Most of the non-linear structures like 6 also form hexagonal columns. Banana-shaped amphiphiles (7) and elongated forks (8) are borderline between lamellar and columnar phases, and their exact textures are determined by environmental conditions. They may also manifest bicontinuous cubic phases. The cone-shape molecule 9 normally displays discontinuous cubic phases. Star-like substituted molecules such as 10 will prefer columnar phases. If the star structure is dissymmetric as in 11, rectangular and tetragonal columnar phases are expected.

