Most common nonionic emulsifiers are ethoxylated alcohols, acids, or oils. The other common nonionic emulsifiers are polyol types, such as sugar ethers (e.g. alkyl glycosides) or sugar esters (e.g. sucrose esters, Figure 1, and sorbitan esters).
Compared to their ethoxylated counterparts, polyol types of nonionic emulsifiers do not lose water solubility with increasing temperature.
Furthermore, sugar esters display many other proven advantages, including robust emulsifying capacity, great stability under stress, and good consumer health.
Sugar esters also demonstrate better biodegradability versus their ether counterparts.
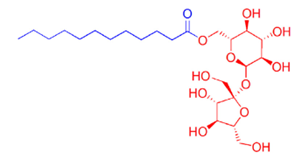
A representative structure for sucrose esters (left).
Sorbitan esters are well-established products mainly used as leather and textile auxiliaries or as emulsifiers for food; their annual global consumption is about 20,000 tons.
Sucrose esters are relatively hydrophobic products with a market size of approximately 5,000 tons per year; they are used as emulsifiers for food and cosmetics.
In comparison, alkyl polyglycosides have a total market size of about 100,000 tons per year and are mainly used for cosmetic, manual dishwashing, and detergent applications.
Due to their natural raw material origin, good performance, and mildness, alkyl polyglycosides are the most successful sugar-based surfactants nowadays.
Sugar esters are nontoxic, compatible with skin, low or non-irritant, and generated from renewable resources, making them highly attractive to the cosmetic industry.
Although the melting point of sugar is high, the melting point of sugar esters can vary between 40 and 80 °C, depending on the degree of esterification.
Moreover, the length of the hydrophobic chain and the size of the hydrophilic group on sugar esters provide a wide range of HLB values. (High HLB values represent water-soluble surfactants, and low HLB values represent oil-soluble emulsifiers; the values can range between 0 and 16.)
Longer fatty acid chains and higher degrees of esterification result in lower HLB values.
For instance, if all eight hydroxyl groups in sucrose were to be esterified, the product would be highly hydrophobic and soluble in oil.
However, partial esterification would generate an amphiphilic sugar ester, which could be used as an emulsifier in the food, cosmetic, and pharmaceutical industries.
Amphiphilic sugar esters can form thermodynamically stable molecular aggregates called micelles in an aqueous solution. The sugar ester’s molecular structure and the experimental conditions influence the critical micelle concentration (CMC) value, which is the specific sugar ester concentration at which micelles start forming.
In general, increasing the alkyl chain length decreases the CMC value. The CMC value is highly relevant as it represents the amount of sugar ester required to solubilize hydrophobic compounds in water. Adding more sugar ester after the CMC is reached yields more micelles and promotes the growth of aggregates.
Sugar esters are capable of effectively reducing the surface tension of water, which is highly valuable for many industry applications. For instance, coconut milk, as an emulsion, is not stable, and its phases separate rapidly. Coconut milk emulsions have larger droplet sizes and lack good emulsifiers, which lead to unfavourable contact between water and oil.
Using emulsifiers capable of reducing surface tension improves emulsion stability. The greater the ability of an emulsifier to reduce surface tension, the greater the emulsion stability formed in coconut milk.
Research conducted on a series of sugar esters including fructose, sucrose, and lactose esters demonstrated that lactose esters are the best molecules among the group; they reduced surface tension from 52.0 to 38.0 N/m, displaying an emulsification index of 54.1%.
Decreasing surface tension is also relevant to generating foods which consist of foam, because the air/water interfacial area can be enlarged by decreasing surface tension.
The biodegradability of sugar esters is also a relevant factor as it helps determine if the concentration of sugar esters remains below levels that are detrimental to the environment. For example, household cleaning products, which contain surface active molecules such as sugar esters, are normally disposed of through the drain.
Therefore, the biodegradability of sugar esters becomes of interest as the detergent surfactants’ residues are linked to foaming incidents in sewage treatment plants.
However, the biodegradability of nonionic surfactants is more difficult to predict because of the wide variety of molecular structures and the lack of a common functional group.
In general, sugar esters are known for their excellent biodegradability and do not generate environmental pollution. Researchers have found that changes to the sugar head group size, the alkyl chain length, and the number of alkyl chains attached to one sugar head group had no significant effect on the biodegradability of sugar esters.
The only structural variation that did have a significant effect was the presence of side groups on the alkyl chain adjacent to the ester bond. These branched groups decreased the rate and extent of the biodegradation of the sugar esters.
This effect was greatest in the presence of an α-sulfonyl group. When α-ethyl or α-methyl groups were attached, the inhibitory effect was weaker but still significant.
In summary, sugar esters are relevant as emulsifiers to the scientific and industrial communities.
However, many questions remain for scientific research and applied studies regarding the nature of these compounds.
Their wide range of possible HLB values provides room for the design and synthesis of novel sugar esters for food, pharmaceutical, and cosmetic applications.
Feeling inspired?
Then why not visit one of the in-cosmetics events around the world?


