In a recent contribution, we discussed how skincare actives can be derived from food and absorbed through skin. This application is logical, since if we consume a species as food, then the chance that we encounter phytotoxicity when applying it as a cosmetic is smaller.
Because many plants produce toxic substances in self-defense, it is essential to understand a plant’s phytotoxins thoroughly before using that particular plant in food or cosmetics. Selecting cosmetic actives based on known food-safe plants reduces the probability of encountering phytotoxins; however, just because a substance is safe to eat does not mean it is safe to apply to skin. This is because, when applied to skin, these substances encounter different environmental conditions than when consumed as food. The most significant difference between the food environment and the skin environment is the prevalence of sunlight when plant-based actives are applied to skin.
Phytotoxicology is the study of plant poisons, which are part of a plant’s defense system. Most of the poisonous higher plants are angiosperms (flowering plants), though only a small percentage of them are recognized as poisonous. Poisonous plants can be classified by the chemical nature of their toxic constituents, their phylogenetic relationships, their botanical characteristics, etc.; however, a classification based on toxic effects is most useful to consumers. According to this approach, poisonous plants can be classified into four families:
- Plants that are poisonous when eaten.
- Plants that are poisonous upon contact.
- Plants that produce airborne allergies.
- Plants that produce photosensitization.
In the cosmetic industry, we usually start our search for plant-based actives within species that have been proven safe by the food industry. As such, poisonous plants in families 1-3 are not considered for cosmetic applications. In the food industry, plant ingredients are not exposed to excessive sunlight before they are consumed, so photosensitization is not usually an item of concern there. It is, however, of great importance when plant ingredients are used on skin, particularly for skin applications where sunlight exposures are expected.
Photosensitization is caused by non-thermal-reactive molecules called photosensitizers (PSs). While these molecules are harmless in the absence of sunlight, they display cytotoxic activities under light irradiation. Because of this behavior, their potential toxicities can escape detection until they are used in applications where light exposures are present.
Herbal plants and plant extracts are natural compounds that are generally regarded as green when compared to synthetic chemicals. Because of their environmental sustainability and reduced side effects, natural substances and herbal actives are becoming the preferred choice to satisfy many cosmetic performance requirements. Understanding the phototoxicity of plant compounds, particularly compounds which are otherwise safe for food applications, is critical to successfully adopting these compounds as cosmetic active ingredients.
To demonstrate the mode of action of a PS, the photophysical and photochemical background of photosensitization is explained by way of a classical Jablonski diagram (Figure 1). When a PS is at ground state, it has no dark toxicity. When exposed to light of appropriate wavelengths, the PS is excited into the S1 state, from which it can fluoresce and return to ground state or undergo ISC to the T1 state. When at the T1 state, the PS can phosphoresce and return to ground state, react directly with other biomolecules (Type I photosensitization), or transfer the excited energy to ground state oxygen (Type II photosensitization). During both Type I and Type II photosensitization, toxic compounds, primarily reactive oxygen species (ROS), are generated; these compounds can then trigger a deadly cascade of cells.
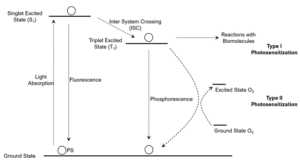
Figure 1: The Jablonski diagram and photosensitization processes.
As such, photosensitization needs to be considered when selecting plant-based actives for cosmetic applications. Some common plants with no dark toxicity contain potent PS molecules; in the absence of light, these plant materials are very beneficial as food or cosmetic ingredients (Table 1). However, because of their known PS components, natural cosmetic ingredients derived from these plants should be limited to applications where no significant light exposures are expected.
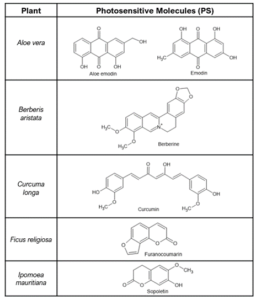 Table 1: Plants and their photosensitizer ingredients.
Table 1: Plants and their photosensitizer ingredients.
To exemplify the importance of considering the photosensitivity of food ingredients before using them in cosmetics, let us take the food colorant curcumin as an example. Curcumin is labeled as E100 and found in the rhizome of Curcuma longa. The class of curcuminoids consists of linear diarylheptanoids, which are themselves composed of four aglycons—dicinnamoylmethane, curcumin, demethoxycurcumin, and bisdemethoxycurcumin (Figure 2). Curcumins have a versatile pharmacological activity spectrum and are shown to provide many health benefits when consumed as food. When used as cosmetic ingredients, similar antioxidizing performances have also been reported. However, because of the well-known photosensitivity of the curcuminoid structure, curcumins or Curcuma longa extract should not be recommended for cosmetic applications related to sun exposure; the resulting buildup of toxic compounds could damage or even kill cells.
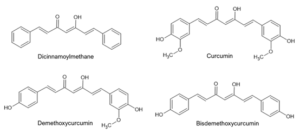
Figure 2: Structures of curcuminoids.
Enjoyed this article? Get more by subscribing to our newsletter!

