Recently, we explored how the science of “watching paint dry” can significantly improve sunscreen performance without additional UV filter usage. For skincare product formulation, in addition to guaranteeing the desired skin feel during the entire time the cosmetic interacts with skin, an optimized formula provides a dynamic environment for critical skincare ingredients such as aromas, actives, and water (Figure 1). Accurately transferring these ingredients to, and maintaining them at, their “sites of engagement” for the desired duration is the soul of skincare product formulation.
Managing the drying of a skincare product—the rate and degree of water transition from the applied cosmetic to the air and skin—is essential to its moisturization performance. Furthermore, the rate and degree of evaporation of fragrance molecules control the intensity and longevity of the product’s aroma. Most significantly, understanding how a skincare formula dries allows us to maintain appropriate solubility of skincare actives during the cosmetic’s dynamic lifetime of being applied to skin.
Maintaining proper active concentrations in the applied cosmetic is critical to the efficacy of the skincare actives. Concentrations need to be high enough to achieve the osmotic pressure necessary to drive the actives to “sites of engagement.” However, concentrations that are too high induce precipitation of the actives, making them unavailable for skin penetration.
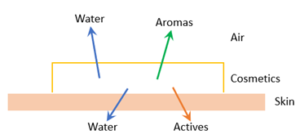
Figure 1: The drying process of a cosmetic product.
Emulsions are the predominant vehicles for delivering actives to the skin. Just as wet paint in a can is very different from dried paint on a wall, a skincare emulsion product in a bottle is very different from the product on skin; this is because, like paint, the skincare product undergoes several structure transformations while drying. However, a thorough review of the associated scientific research is not the purpose of this column. Instead, a simplified overview of the methodology is presented here to illustrate the impact of the drying path on the performance of skincare cosmetics.
For illustrative purposes, we can use a phase diagram to investigate the evaporation path of an oil component (simulating an oil-soluble aroma molecule) under different relative humidity levels. To mimic a common skincare formulation, a simple emulsion with an oil to water weight ratio of more than 1/3 was formed and subjected to evaporation under a wide range of relative humidity (RH) levels. The emulsion was made from water (W), an oil phase (O) with a small water-soluble fraction, and a non-ionic emulsifier (S) with insignificant water solubility while forming liquid crystals (LC) at the O-W interface. The system described is presented in Figure 2.
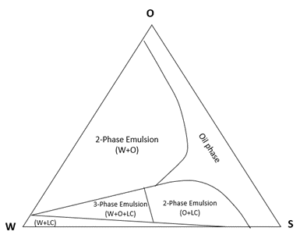
Figure 2: Phase diagram of the emulsion system studied.
In Figure 3, the evaporation paths of a two-phase O/W emulsion (0.65, 0.30, 0.05) are depicted under different relative humidities. At the high O/W weight ratio level (> 1/3), we can see that the relative humidity affects the evaporation path only at exceptionally high RH levels. There are only minor variations in the evaporation path when the RH is increased from 0% to about 90%. From 0% to about 93% RH, emulsion evaporation results in the reduction of the aqueous phase until it is depleted; after that, evaporation is limited to oil component evaporation in the oil phase.
When the RH is at 95%, the evaporation path is significantly different. At this level, the oil phase disappears faster than the aqueous phase, and at a certain stage of the evaporation process, the lamellar liquid crystal alignments of the emulsifier molecules appear in the emulsion. When the RH is at 97.5%, the oil phase disappears even faster, and there is an increase in the aqueous weight of the emulsion. The lamellar liquid crystal also appears in the emulsion during evaporation.
When the weight ratio of O/W is lower than 1/3, RH levels as low as 40% can cause substantial changes in the emulsion’s evaporation path.
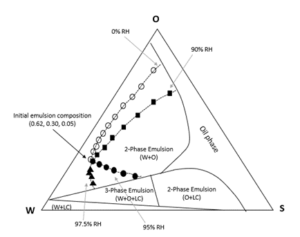
Figure 3: Evaporation paths of an emulsion (W-0.65, O-0.30, S-0.05) under different relative humidity (RH) levels.
It is now widely known that the presence of liquid crystals in an emulsion affects the properties of that emulsion significantly. In addition to altering the rate and degree of evaporation, liquid crystals also change the rheology of the emulsion. Since rheology influences the skin feel and permeance of a cosmetic product on skin, rheological properties—and thereby the drying paths of emulsions—are the keys to managing the quality of cosmetic coverage and the homogenous and uniform distribution of cosmetics on skin.
In summary, cosmetic actives, many of which are derived from the food industry, are soluble in an emulsion’s water or oil phases, depending on their structures. During the drying process, the relative weights of the water and oil phases keep changing, which impacts the concentrations of actives in the cosmetic. Consequently, having a thorough understanding of evaporation paths allows one to design skincare formulations that maximize the availability of actives to skin via controlling their concentrations.
However, drying is only one piece of the puzzle. The molecular structures of emulsifiers profoundly influence the ease of, and emulsion composition space for, liquid crystal formation, and appropriate formation of liquid crystals drives the comprehensive sensory perception of the cosmetic in terms of both skin feel and aroma. Furthermore, appropriate liquid crystal organization is crucial to the efficacy of the cosmetic product with respect to the availability of actives and the comprehensiveness of sun protection. The structure and alignment of emulsifier molecules and their impacts on emulsion properties are topics of our future contribution.
Enjoyed this article? Get more by subscribing to our newsletter!

