Vitamin D– In our efforts to protect skin from damaging UV light, should we be compensating for the lost vitamin D?
Vitamin D (calcitriol) is critical for bone metabolism. Yes, it is common knowledge that insufficient vitamin D will result in bone softening (osteomalacia) leading to rickets, but perhaps less well known is how too little vitamin D (calcitriol) leads to a whole host of clinical conditions and, with COVID-19 in mind, it is important to note that vitamin D deficient people have an increased susceptibility to bacterial and viral infections. When formulating skin care products to nourish and maintain healthy skin, it is well worth remembering that vitamin D is essential for good skin health.
Vitamin D synthesis starts with Sunlight on skin
Vitamin D synthesis mainly takes place in the lower stratum basale and stratum spinosum layer of our skin. UVB in sunlight causes the nonenzymic conversion of 7-dehydrocholesterol to previtamin D3, (which in turn spontaneously isomerises into vitamin D3 (Cholecalciferol)) see figure 1.
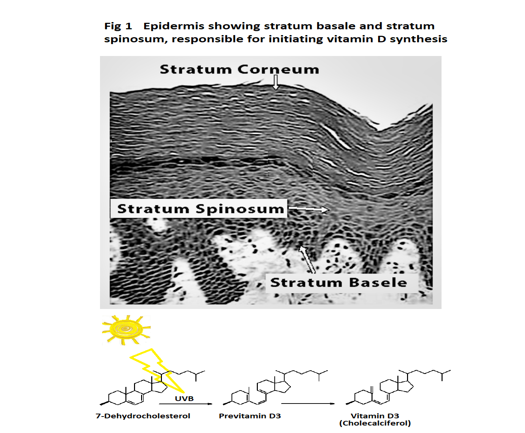
Next, vitamin D3 (Cholecalciferol) is gathered up by vitamin D binding protein (DBP) in plasma. This pulls the equilibrium in favour of skin synthesising more vitamin D3 (Cholecalciferol). DBP is extremely important. It regulates vitamin D3 (Cholecalciferol) synthesis and the levels of both free and total vitamin D metabolites in plasma. DBP acts as a vitamin D metabolite carrier and reservoir for vitamin D metabolites in blood so preventing rapid vitamin D deficiency [1].
Vitamin D (Calcitriol) in general and skin inflammation and stress
The liver metabolises vitamin D3 (Cholecalciferol) into the prehormone 25-hydroxyvitamin D (calcifediol), which then circulates in the blood. Approximately 0.03% of 25-hydroxyvitamin D (calcifediol) is free, 85% is bound to DBP and 15% is bound to albumin. The kidneys are the most industrious organ at converting 25-hydroxyvitamin D (calcifediol) into the active form of vitamin D, 1,25 dihydroxyvitamin D, (known as calcitriol).
Under the control of Parathyroid Hormone (PHT), kidneys produce active vitamin D (calcitriol) in response to falling Ca2+ levels. High plasma levels of active vitamin D (calcitriol) increases the uptake of Ca2+ by the intestine and when plasma Ca2+ levels are returned to normal, PTH levels decrease and kidneys return to baseline active vitamin D (calcitriol) production.
To complicate things further, many other tissues also convert 25-hydroxyvitamin D (calcifediol) into the active form. For example, bacterial products can activate immune cells (monocytes, macrophages and epithelial cells) to trigger local synthesis of active vitamin D (calcitriol), which induces the production of cathelicidin antimicrobial peptides [2].
This source of active Vitamin D (calcitriol) may modulate local inflammation and immunity by supressing the proliferation of T-helper 1 cells, which make interferon g (IFN-g) and IL-2. Active vitamin D (calcitriol) inhibits UVB-induced skin cell death, inhibits the activation of stress-activated protein kinases and the production of the powerful inflammatory cytokine, interleukin-6, by keratinocytes [3].
Partnership: vitamin D and its receptor… and allergy
Active Vitamin D (calcitriol) works at the cellular level through the vitamin D receptor (VDR) regulating gene expression [4]. cDNA microarray analyses of mRNAs indicate that between 500–1000 coding genes may be regulated by the VDR [5]. VDR is found in the cytosol of a diverse range of cell types and is present in virtually every organ including skin.
Interestingly, thinking about skin sensitivity and allergy, VDR is in many inflammatory cells, including activated T and B-lymphocytes, macrophages and dendritic cells. Dendritic cells are abundant in skin. They are the key antigen presenting cells that activate T cells, which, in Type 1 allergies, results in (Th2)-driven specific immune response towards allergens [6]. Note that Th2 cells produce cytokines that contribute to IgE production and allergic responses.
Vitamin D, hair, skin and ageing
Hair follicle cycling and epidermal cell differentiation depend on active vitamin D (calcitriol) and VDR [7]. Studies where VDR was Knocked out resulted in, among other things, dilated hair follicles leading to hair loss. Interestingly, Ca2+ is an important co-regulator with active vitamin D (calcitriol) in epidermal differentiation. Knocking out Ca2+ sensing receptor (CaSR) together with VDR, accelerated the development of skin cancer in animal models. This, and research showing active vitamin D (calcitriol) inhibits UV-induced cell death, indicate that active vitamin D (calcitriol) and VDR are involved in several important skin processes, which if disrupted interfere with differentiation and predispose skin to cancer formation [4]. Skin’s healthspan depends on vitamin D and as we age, we see the concentration of 7-dehydrocholesterol and total previtamin D3 in skin decline so that by the time a 20-year-old has reached 80-years the 7-dehydrocholesterol and previtamin D in their skin will have halved [8].
Vitamin D and the microbiome
As there is insufficient data on how vitamin D affects the skin microbiome, we can only speculate that just as vitamin D status influences the gut and lung microbiomes, it may also affect the skin’s microbiome [9]. It is interesting to note how studies that knocked out intestinal epithelial VDR lead to dysbiosis. Microbes in the gut are known to modulate immunity and allergy, so by effecting changes in the gut microbiome, active vitamin D (calcitriol) may be indirectly influence immune function [10].
As mentioned earlier, bacterial products can activate the local production of active vitamin D (calcitriol) in skin, which then through VDR, brings about the expression of cathelicidin antimicrobial peptides. Cathelicidin will modify the local skin microbiome and the crosstalk between adapting microbiome and cells producing active vitamin D (calcitriol), may reduce inflammation just as is observed in gut and lungs. Microbiome dysbiosis is observed in psoriasis and atopic dermatitis. Interestingly, topical application of active vitamin D (calcitriol) has been found to be effective in treating psoriasis, atopic dermatitis and other inflammatory skin diseases [11].
Therefore, it can be ventured that active vitamin D (calcitriol) may help skin’s microbiome resist dysbiosis and so inhibit inflammatory responses such as seen in psoriasis and other inflammatory diseases [12].
How much vitamin D is your skin making now?
The absolute amount of vitamin D3 (Cholecalciferol) made by skin will depend on how much skin is exposed to sunlight, the strength of the sunlight (so on the location, season, altitude, weather and the time of the day and on the level of skin pigmentation) and also if the skin is protected by sunscreen. Taking these influences into consideration, a good working estimate is when a person with skin type III exposes their hands and face to spring and summer sunshine for up to 20 – 30 minutes their skin will synthesise the daily requirement of 10 μg of vitamin D3 (Cholecalciferol).
Problems arise in the winter when we are wrapping-up against the cold, as at least to 2 hours of midday sun is needed for the same amount of exposed skin to produce 10 μg of vitamin D3 (Cholecalciferol) [13]. Remembering, that the nights are now drawing in and how little time most of us will be spending outdoors in the winter, it is not surprising that most people in the UK are vitamin D deficient. UVB radiation does not penetrate glass, so exposure to sunshine in cars and indoors will not produce vitamin D. A recent UK study found that around 20% of 1,000 office workers spent up to 30 minutes outside and for nearly 40% of them this was just 15 minutes outside a day [14].
Dietary vitamin D
Undoubtedly, vitamin D in our diet is important and although the numbers of children suffering with rickets has increased, good dietary advice, supplements and fortified foods, have kept rickets at bay for decades. However it should be noted that as far back as in the mid-17th century, Francis Glisson, Professor of Physics at Cambridge University, observed that rickets was common among infants and young children of country farmers who ate well, and whose diets were known to include eggs and butter, but who lived in rainy, misty parts of the country and who were kept indoors during long severe winters [15].
As Professor Glisson’s observation suggests, vitamin D in food sources are poorer alternatives to the vitamin D produced when sunshine penetrates skin. There are only a few foods, which naturally contain vitamin D. Dietary sources of vitamin D include milk, fish, fish liver oils, liver, red meat, eggs, oysters, caviar, honey and bean sprouts [16][17]. Certain microalgae contain both provitamin D3 and vitamin D3 (Cholecalciferol). Some fungi and yeast contain ergosterol, which can be converted to vitamin D2 (ergocalciferol) by UVB irradiation. Ergosterol in plant material is normally due to fungal contamination. Note that liver needs nearly twice as much vitamin D2 (ergocalciferol) as vitamin D3 to make 25-hydroxyvitamin D (calcifediol).
Intriguingly, a few calcinogenic plants, (yellow oat grass Trisetum flavescens, Solanum malacoxylon, Cestrum diurnum, and Nierembergia veitchii of the Solanaceae family) are toxic to grazing animals because they contain vitamin D glycosides, which induce calcinosis when gut microbes, hydrolyse the glycosides, releasing active vitamin D (calcitriol). This excess of active vitamin D (calcitriol) in the animal, stimulates the over synthesis of calcium binding protein (CaBP) in the intestinal mucosa. CaBP transports digested calcium and phosphate from the intestines into the blood so causing hypercalcemia. The excess absorbed mineral is then deposited in soft tissues causing calcinosis [19].
Other plants that contain Vitamin D metabolites include members of the Cucurbitaceae, Fabaceae, and Poaceae and certain plants which strongly associated with fungus or those that naturally produce 7-dehydrocholesterol such as wheat germ oil, which if subject to UVB radiation, should in theory contain vitamin D precursors [20].
Vitamin D as a cosmetic ingredient
Several ingredients in the vitamin D family are described as having antioxidant and skin-soothing benefits. 7-dehydrocholesterol is listed on COSing as an emulsion stabilising, skin conditioning and viscosity controlling ingredient suitable for EU cosmetics, however, active vitamin D (calcitriol) is a drug so it is not considered a cosmetic ingredient and both ergocalciferol (vitamin D2) and cholecalciferol (vitamins D3) are on the list of substances prohibited in EU cosmetics. (They are number 335 on Annex II of the EU. Cosmetics Regulations).
A quick search of in-cosmetics ingredient database, which can be found on www.in-cosmetics.com/products/ingredients-database/, as well as looking through UL Prospector and on Chemberry data bases, results in three suppliers of 7-dehydrocholesterol. There are also suppliers of cholecalciferol, either in vitamin blends or in vegetable carrier oils. Plant-derived active ingredients claimed to stimulate the expression of VDR are available for moisturisation. Avocado oil with vitamin D at 80.000 IU/kg is available from one supplier. It is worth mentioning in passing that 1 μg of vitamin D is equal to 40 IU and daily dietary intakes of 600-800 IU is recommended for adults https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
A shitake mushroom extract that provides anti-inflammatory, anti-bacterial and free radical scavenging is recommended for antiaging creams, sensitive skin care to reduce irritancy and to stimulate self-protection contains many things including vitamin D. Caviar extracts are also available which contain vitamin D.
Summary and apology
It was necessary to belabour the detail of vitamin D metabolism to show just how vital skin’s role is in initiating vitamin D synthesis, how important vitamin D is for healthy skin and how, it is more than likely that active vitamin D (calcitriol) is necessary for a healthy skin microbiome.
Because dietary vitamin D is a poor substitute for the active vitamin D (calcitriol) produced naturally in skin by sunlight, the cosmetic industry really should consider vitamin D when formulating products. The problem is a little sunlight is essential but excessive exposure to UV light damages skin and can cause carcinomas. Just 5 to 10 mins exposure to sunlight starts becoming harmful to people with very fair skin so should and can, high SPF products compensate for the reduction in vitamin D they cause? Could topical 7-dehydrocholesterol make up for the loss of 7-dehydrocholesterol as we age? Then there is COVID-19.
As I completed this article, a publication reported observations indicating that ”The severity of clinical outcomes from COVID-19 and mortality were reduced in patients who were vitamin D sufficient.” i.e. with normal 25-hydroxyvitamin D (calcifediol) serum levels of ≥ 30 ng/mL [22]. So, now that the nights are drawing in and we are wrapping-up against the cold, spare a thought for your skin and for vitamin D, it could be a matter of life or death.
Why not check out our latest ingredient review video?
References
- Bouillon, R., Frans, S., Leen, A., and Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. (2020) Front. Endocrinol. 10. p910. www.frontiersin.org/article/10.3389/fendo.2019.00910
- Gombart, A. F. (2009). The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future microbiology, 4(9), 1151–1165. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2821804/
- De Haes, P., Garmyn, M., Degreef, H., Vantieghem, K., Bouillon, R., Segaert, S. (2003) 1,25-Dihydroxyvitamin D3 inhibits ultraviolet B-induced apoptosis, Jun kinase activation, and interleukin-6 production in primary human keratinocytes. J Cell Biochem. v89:663–73.
- Bikle, D. D., Oda, Y., Tu, C. L., & Jiang, Y. (2015). Novel mechanisms for the vitamin D receptor (VDR) in the skin and in skin cancer. The Journal of steroid biochemistry and molecular biology, 148, 47–51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4361259/
- Reichrath, J., Reichrath, S., Heyne, K., Vogt, T., & Roemer, K. (2014). Tumor suppression in skin and other tissues via cross-talk between vitamin D- and p53-signaling. Frontiers in physiology, 5, 166. https://doi.org/10.3389/fphys.2014.00166
- Humeniuk, P., Dubiela, P., & Hoffmann-Sommergruber, K. (2017). Dendritic Cells and Their Role in Allergy: Uptake, Proteolytic Processing and Presentation of Allergens. International journal of molecular sciences, 18(7), 1491. https://doi.org/10.3390/ijms18071491
- Haussler MR, Whitfield GK, Kaneko I, et al. (2013) Molecular mechanisms of vitamin D action. Calcif Tissue Int. v92(2):77-98. https://pubmed.ncbi.nlm.nih.gov/22782502/
- Chang AL, Fu T, Amir O, Tang JY. (2010) Association of facial skin aging and vitamin D levels in middle-aged white women. Cancer Causes Control. 21:2315–6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3583891/
- Wang J, et al., (2016) Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet, v48(11): p. 1396–1406 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5626933/
- Wu S, et al., (2015) Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut, v64(7): p. 1082–94.
- Kircik. L (2009). Efficacy and safety of topical calcitriol 3 microg/g ointment, a new topical therapy for chronic plaque psoriasis. Journal of Drugs in Dermatology. 8 (8 Suppl.): s9 16. https://pubmed.ncbi.nlm.nih.gov/19702031/
- Sherwani, M.A., Tufail, S., Muzaffar, A.F. and Yusuf, N. (2017). The skin microbiome and immune https://doi.org/10.1111/phpp.12334
- Serrano, M., Cañada, J., Moreno, J.C., and Gurrea, G. (2017) Solar ultraviolet doses and vitamin D in a northern mid-latitude. Science of The Total Environment, p574: 744 DOI: 10.1016/j.scitotenv.2016.09.102
- Clarke, R. May 18, (2018). HR Strategy News. https://www.hrreview.co.uk/hr-news/strategy-news/40-of-brits-spend-just-15-minutes-outdoors-each-day/111130
- Holick M.F., Vitamin D. In: Modern nutrition in health and disease. Shils, M.E., Olson J.A., Shike, M., Ross A.C., editors. Lippincott; Williams & Wilkins (1999). pp. 228–239.
- 2015-2020 Edition of the Dietary Guidelines for Americans. Appendix 12. Food Sources of Vitamin D https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/appendix-12/
- Kim, T.-K.; Atigadda, V.; Brzeminski, P.; Fabisiak, A.; Tang, E.K.Y.; Tuckey, R.C., and Slominski, A.T. (2020) Detection of 7-Dehydrocholesterol and Vitamin D3 Derivatives in Honey. Molecules, 25, 2583. https://www.mdpi.com/1420-3049/25/11/2583
- Safety Assessment including Current and Emerging Issues in Toxicologic Pathology
- Bryan L. Stegelmeier, B.L., and Cook, D. Calcinogenic Glycoside-Containing Plants. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology (Third Edition), 2013
- Baur, A. C., Brandsch, C., König, B., Hirche, F., & Stangl, G. I. (2016). Plant Oils as Potential Sources of Vitamin D. Frontiers in nutrition, 3, 29. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4981617/
- J.Y. Tang, T.Z. Xiao, Y. Oda, K.S. Chang, E. Shpall, A. Wu, et al. (2011). Vitamin D3 inhibits Hedgehog signalling and proliferation in murine Basal cell carcinomas. Cancer Prev. Res, 4, pp. 744-751.
- Maghbooli, Z., Sahraian, M.A., Ebrahimi, M., Pazoki, M., Kafan, S., Tabriz, H.M., Hadadi, A. Montazeri, M., Nasiri, M., Shirvani, A., and Holick, M.F.Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. Published: September 25, 2020. https://doi.org/10.1371/journal.pone.0239799

