Previously, we established that plant-based natural food materials are rich sources of skincare actives. In this article, we will dig deeper into these naturally occurring molecules by describing their antioxidative mechanisms and properties.
Antioxidation is important to cell survival. Normally, reactive oxygen species (ROS) are produced via neutrophil activation and are used by phagocytes to kill infectious agents. However, an overproduction of ROS can cause oxidative stress and cellular damage; it is thereby the leading cause of degenerative diseases. Human skin is particularly susceptible to ROS overproduction, which may be caused by both endogenous sources and environmental factors, as shown in Figure 1.
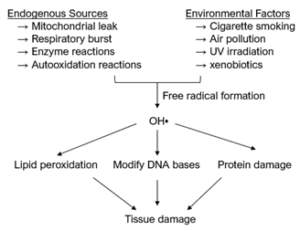
Since plants are also vulnerable to ROS overproduction, they, like humans, rely on antioxidants for cell protection. Antioxidant molecules are produced in many parts of plants and are closely associated with plant evolution. Plants use antioxidants to overcome two major threats to their survival: solar oxidative stresses and biological stresses.
To combat solar oxidative stresses, plant antioxidants use three distinguishable mechanisms:
- The absorption mechanism, which is employed by antioxidant molecules that are light absorbers. These molecules absorb sunlight directly when the photosynthesis unit experiences excessive sunlight exposure.
- The quenching mechanism, which is employed by antioxidant molecules that are quenchers. These molecules accept excited state energy from chlorophylls, thus preventing the chlorophylls from suffering solar stresses.
- The neutralization mechanism, which is employed by antioxidant molecules that are neutralizers. When the first two mechanisms fail and ROS are produced as a result of oxidative stress in the photosynthesis unit, these molecules neutralize the ROS (usually by acting as reducing agents and being oxidized themselves) and therefore prevent ROS from harming the cell.
To combat biological stresses, plants produce antioxidants that make them less attractive to herbivores and pathogens. These antioxidants also prevent pathogens from reproducing on the plants. For example, neutralizers can create high chemical oxygen demand (COD) so that pathogens or other undesirable organisms cannot grow on or around the plants.
Like their antioxidative mechanisms, plant antioxidants can be grouped into three families: (1) phenolics, which include simple phenols and polyphenols, (2) terpenes and multi-terpenes, and (3) molecules without phenol or terpene structures.
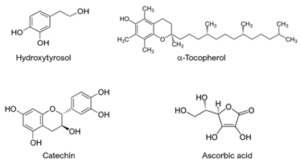
Phenolic antioxidant molecules almost exclusively use the neutralization mechanism. They donate hydrogen atoms to reactive radical species and act as chain breakers to stop oxidation. Typical plant antioxidants in this family include hydroxytyrosol, α-tocopherol (a form of vitamin E), and catechin (Figure 2). Though not a phenolic structure, Vitamin C, also known as ascorbic acid, employs the same antioxidative mechanism because of the active hydrogens in its structure.
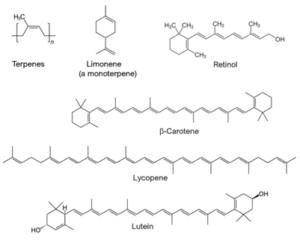
Terpenes are natural, unsaturated hydrocarbons with formulas (C5H8)n, where n > 1, and are classified by number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), and tetraterpenes (C40), for example (Figure 3).
Individual terpene structures are easily oxidized at the unsaturated double bond and thus can function as antioxidants via the neutralization mechanism. Because of their low excited state energy, conjugated terpenes—such as vitamin A (retinol, β-carotene), lycopene, and lutein—can also use the quenching mechanism to protect cells. They quench the excited states of light-absorbing molecules as well as the singlet excited states of oxygen molecules.
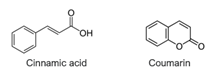
Antioxidant molecules without phenol or terpene structures use the absorption mechanism. They absorb sunlight to avoid overwhelming the photosynthesis unit, thereby preventing the generation of oxidative stresses. When produced by plants, these molecules are typically based on cinnamate or coumarin structures, as shown in Figure 4.
Enjoyed this article? Get more by subscribing to our newsletter!
Feeling inspired to see ingredients and trends in action?
Then why not visit one of the in-cosmetics events around the world?

